BACME NAT test offers immediate virus discrimination solution of HIV-1, HIV-2, HCV and HBV in a single test tube.
CFDA S20130011
Four-in-One NAT Test
BACME NAT test offers immediate virus discrimination solution of HIV-1, HIV-2, HCV and HBV in a single test tube.
Intended use
The multiplex, real-time PCR test is intended for use to screen donor samples for HIV-1 Group M RNA, HIV-1 Group O RNA, HIV-2 RNA, HCV RNA and HBV DNA in plasma specimens from individual human donors.
BACME Biotech NAT system is composed by two technology platforms: NA Magnetic-bead extraction and multiplex Real-Time PCR platform.
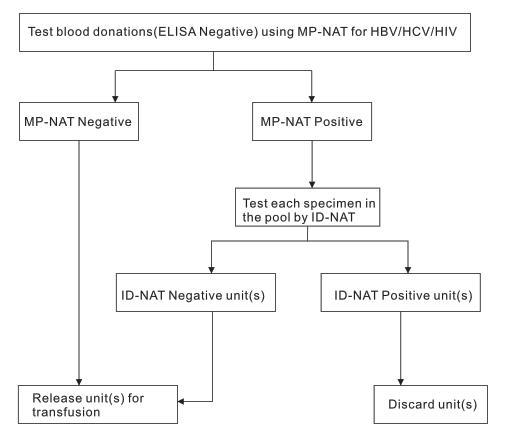
Plasma from all donors may be screened as individual specimens. For donations of whole blood and blood components, plasma specimens may be tested individually or in pools comprised of aliquots of individual specimens in conjunction with serology tests for HIV, HCV and HBV. For an individual specimen, results are simultaneously detected and discriminated for HIV, HCV and HBV.
Dozens HIV and HCV ELISA-negative donations proved to be NAT positive with BACME NAT system every year, as well as thousands of HBV cases.
Testing was done in MP (mini-pools) of size eight or ID (individual donation) test.
This test is not intended for use as an aid in diagnosis of infection with HIV, HCV or HBV.
Specification
Ø One test, four results
Immediately Detects and discriminates HBV,HCV,HIV-1 and HIV-2 in donations from human whole blood in one test.
Ø High Sensitivity and subtypes
Virus | Sensitivity | Subtypes |
HIV-1 | 42.3IU/mL | M,O,N |
HIV-2 | Genotype A CAM2 | |
HCV | 12.5IU/mL | 1,2,3,4,5,6 |
HBV | 4.2IU/mL | A,B,C,D,E,F,G,H |
Ø Double IC:
Both RNA (HCV/HIV) and DNA(HBV) IC
Ø High Efficient:
Mini-Pool Test (from 1 to 8)
Individual Donation Test
Ø Contamination Proof
Using UNG enzyme+dUTP to avoid contamination
MP-NAT Procedure

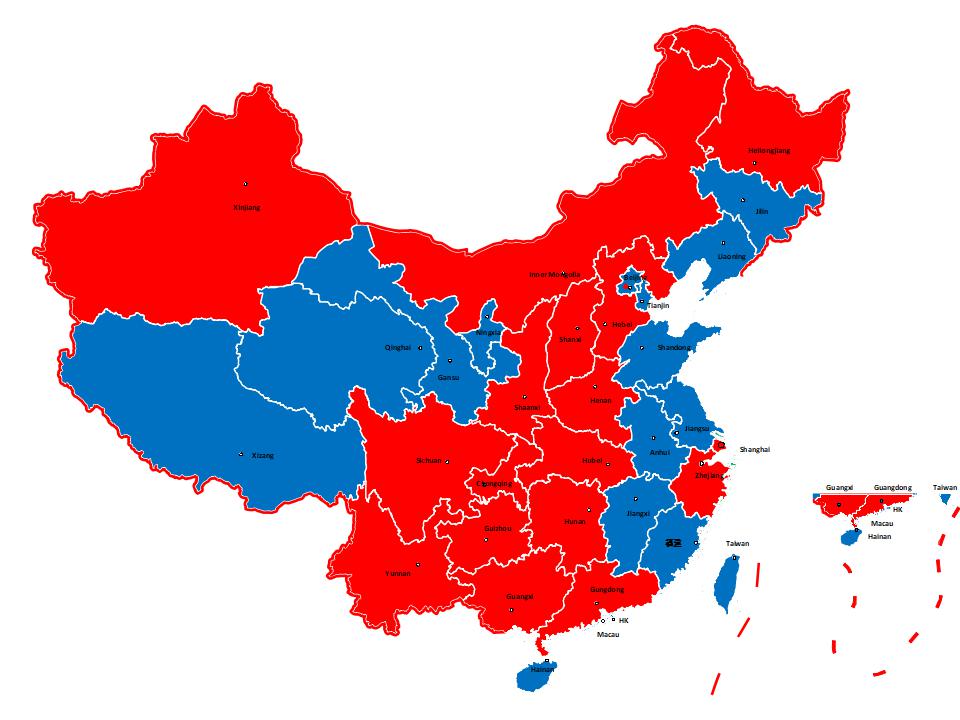
Major Customers in China
■BACME in China