On April 6, 2020, U.S FDA(U.S Food & Drug Administration) issued Pre-Emergency Use Authorization.(Pre-EUA) for BACME Novel Coronavirus Sars-Cov-2 RNA Real-time Kit, which means BACME product can be freely sold in U.S.A.

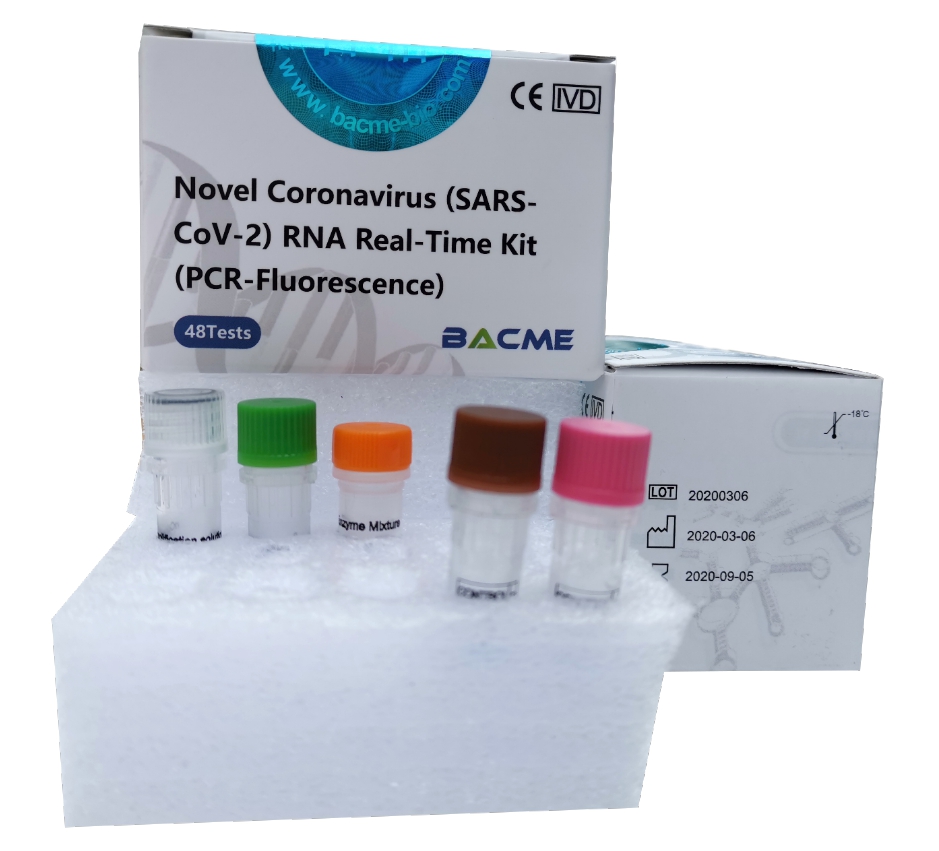
BACME Novel Coronavirus Sars-Cov-2 RNA Real-time Kit
This kit can be used in vitro to qualitatively detect suspected cases of nCoV infection, patients with suspected clustering cases and other nCoV infection diagnostic or differential diagnostic such as ORF1ab, E-gene and N-gene in swab, sputum and alveolar lavage fluid samples.
This Sars-Cov-2 RNA Real-time Kit has passed by CE and got the acknowledgement of FDA, it will help BACME Novel Coronavirus products use for more oversea countries to contribute for the precise control and prevention of global pandemic.